What Is The Function Of A Buffer In Chemistry
NEET Chemistry is divided into three sections namely Physical Organic and Inorganic Chemistry. The phosphate buffer only plays a minor role in the blood however because H 3 PO 4 and H 2 PO 4 - are found in very low concentration in the blood.
 Introduction To Buffers Chemistry Libretexts
Introduction To Buffers Chemistry Libretexts
Compared to Biology and Physics Chemistry is considered to be the most scoring section.
What is the function of a buffer in chemistry. Buffer systems are made of either a weak acid and its salt or a weak base and its salt. Hemoglobin also acts as a pH buffer in the blood. Make interpersonal chemistry a priority Our sense of engagement and satisfaction at work results in large part from the hundreds and hundreds of daily interactions we have while there whether with a supervisor colleagues or customers.
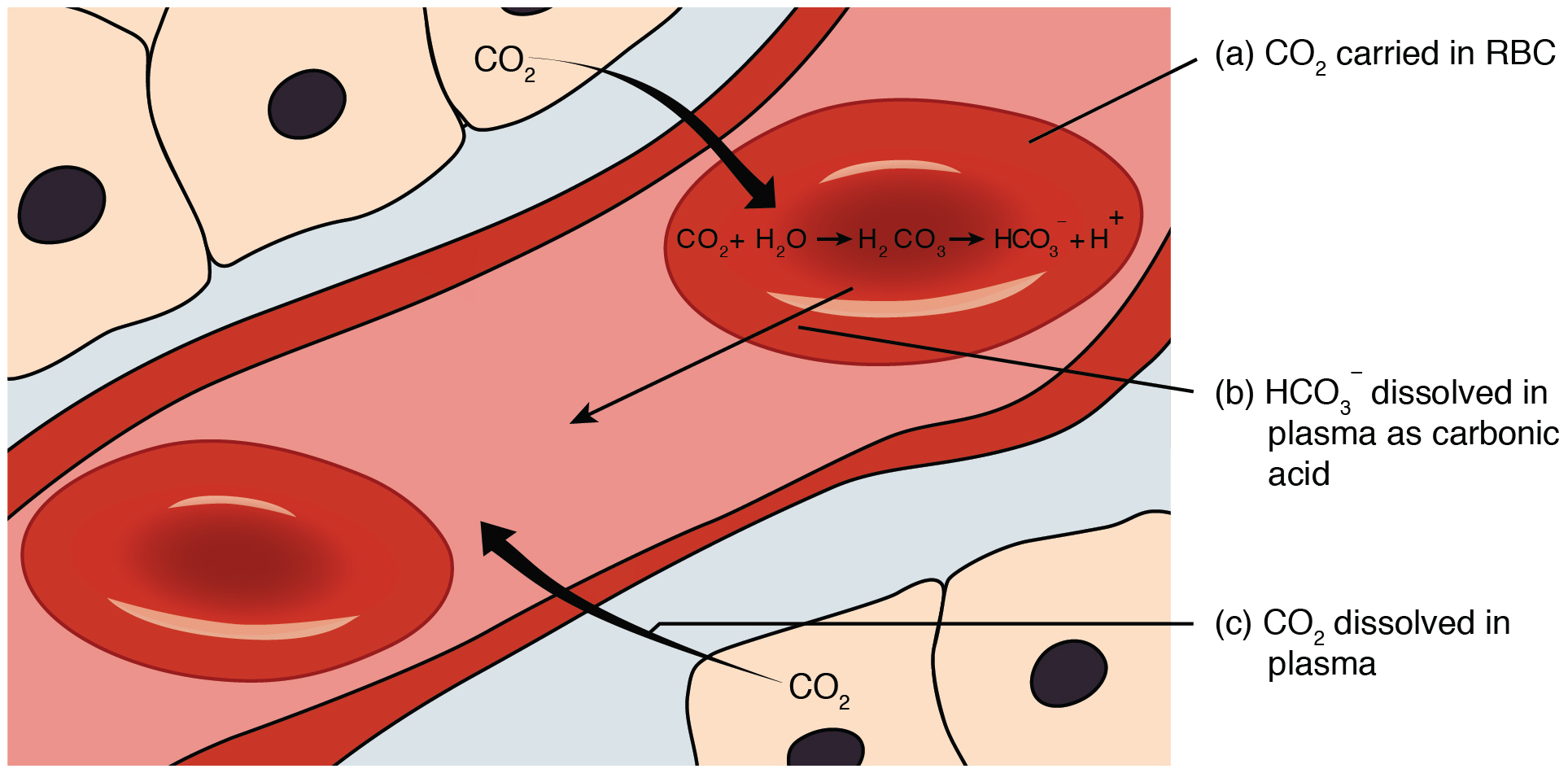
Proteins Peptides Amino Acids 1. Proteins from the Greek proteios meaning first are a class of organic compounds which are present in and vital to every living cellIn the form of skin hair callus cartilage muscles tendons and ligaments proteins hold together protect and provide structure to the body of a multi-celled organism. The pK for the phosphate buffer is 68 which allows this buffer to function within its optimal buffering range at physiological pH.
A lot of biological and chemical reactions need a constant pH for the reaction to proceed. You can implement your own. Itoa is not a standard C function.
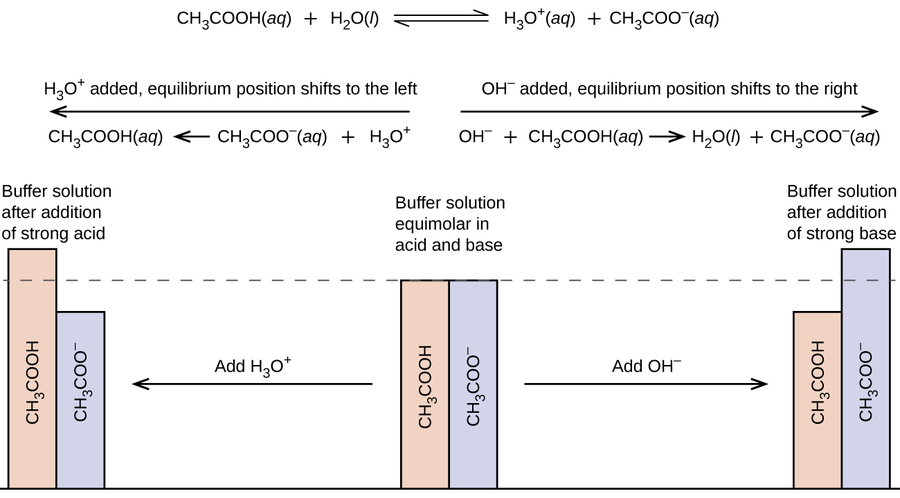
Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. Buffers function best when the pK a of the conjugate weak acid used is close to the desired working range of the buffer. Buffers are extremely useful in these systems to maintain the pH at a constant value.
A buffer solution more precisely pH buffer or hydrogen ion buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versaIts pH changes very little when a small amount of strong acid or base is added to it. Because of its neutral range tris is a commonly used buffer in biological labs. This open-access online general chemistry video repository offered not-for-credit and free of charge from UC Berkeley provides students an introduction to the world of chemistry as seen from a broad variety of perspectives.
It appeared in the first edition of Kernighan and Ritchies The C Programming Language on page 60The second edition of The C Programming Language KR2 contains the following implementation of itoa on page 64The book notes several issues with this implementation including the fact that it does not correctly handle the most. Hemoglobin also acts as a pH buffer in the blood. The Hammett acidity function H 0 is a measure of acidity that is used for very concentrated solutions of strong acids including superacidsIt was proposed by the physical organic chemist Louis Plack Hammett and is the best-known acidity function used to extend the measure of BrønstedLowry acidity beyond the dilute aqueous solutions for which the pH scale is useful.
Trishydroxymethyl aminomethane with a pKa of 81 is an effective buffer between pH 7 and 9. I am reading some data from a file using read. Buffers are solutions that resist a change in pH on dilution or on addition of small amounts of acids or alkali.
Your central nervous system controls and coordinates everything you do including muscle movement organ function and even complex thinking and planning. The brain and spinal cord make up your central nervous system. Find articles on topics such as metabolic pathways and enzymology biochemical structures and sequences genome databases and more.
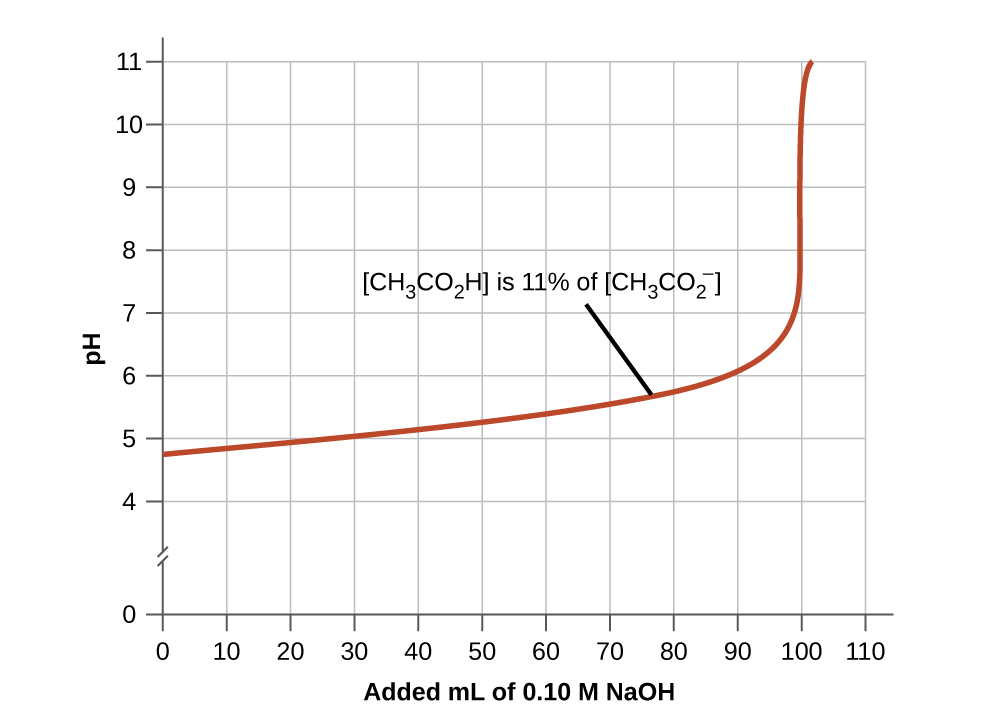
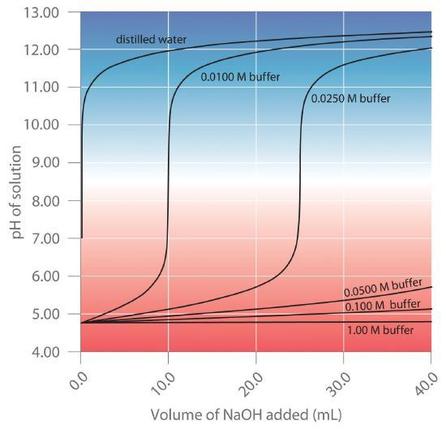
First make the graph of the function then take the modulus Here at the place of sinx we have 2 sinx π4 so the graph will be of type sin x only but the graph will be starting from x -π4. This turns out to be the case when the concentrations of the conjugate acid and conjugate base are approximately equal within about a factor of 10. Just read character by character and do not worry about the exceptions because the condition in the while loop is handling the exceptions D.
E The student proposes that the rate law is. CHEMISTRY EQUATIONS AND CONSTANTS-4- GASES LIQUIDS AND SOLUTIONS. With significant funding from the Camille Henry Dreyfus Foundation we have created studio-quality video segments based on Chem 1A a traditional large.
The pK for the phosphate buffer is 68 which allows this buffer to function within its optimal buffering range at physiological pH. Khyber Pakhtunkhwa Public Procurement Regulatory Authority KPPRA is an autonomous organization established under an Act of the Provincial Assembly of Khyber Pakhtunkhwa. However tris buffer is temperature sensitive and should be used at the temperature at which it was originally pHed to avoid inaccuracy.
Lets Know About KPPRA. The phosphate buffer only plays a minor role in the blood however because H 2 PO 4-and HPO 4 2-are found in very low concentration in the blood. Making a priority of.
Include the net ionic equation for the reaction that occurs when the student adds. Its no secret that culture is a huge part of how we hire new employees at Buffer. Biochemistry applies chemistry concepts to the study of living organisms and the atoms and molecules that comprise them.
Concentration of urea as a function of time as shown by the data table and the graph below. Here I am reading data in a 2d char pointer but the method is the same for the 1d also. Cerebrospinal fluid CSF is a clear colorless liquid found in your brain and spinal cord.
If prepared thoroughly chemistry can help students to secure a meritorious position in the exam. QA for scientists academics teachers and students in the field of chemistry Stack Exchange Network Stack Exchange network consists of 176 QA communities including Stack Overflow the largest most trusted online community for developers to learn share their knowledge and build their careers. A buffer system is a solution that resists change in pH when acids or bases are added to it.
 Bicarbonate Buffer System Wikipedia
Bicarbonate Buffer System Wikipedia
 8 9 Buffer Capacity And Buffer Range Chemistry Libretexts
8 9 Buffer Capacity And Buffer Range Chemistry Libretexts
 Acid Base Balance Anatomy And Physiology Ii
Acid Base Balance Anatomy And Physiology Ii
 Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Buffers Indicators Acids And Bases 101 The Basics Of Chemistry
 Applications Of Buffer In Industries
Applications Of Buffer In Industries
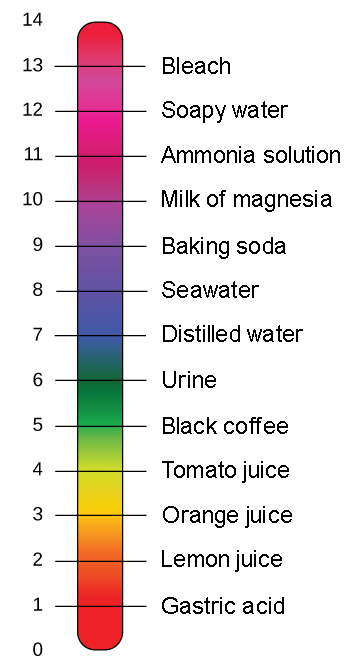 Buffers Ph Acids And Bases Biology For Non Majors I
Buffers Ph Acids And Bases Biology For Non Majors I
 7 1 Acid Base Buffers Chemistry Libretexts
7 1 Acid Base Buffers Chemistry Libretexts
 Ways To Get A Buffer Solution Video Khan Academy
Ways To Get A Buffer Solution Video Khan Academy
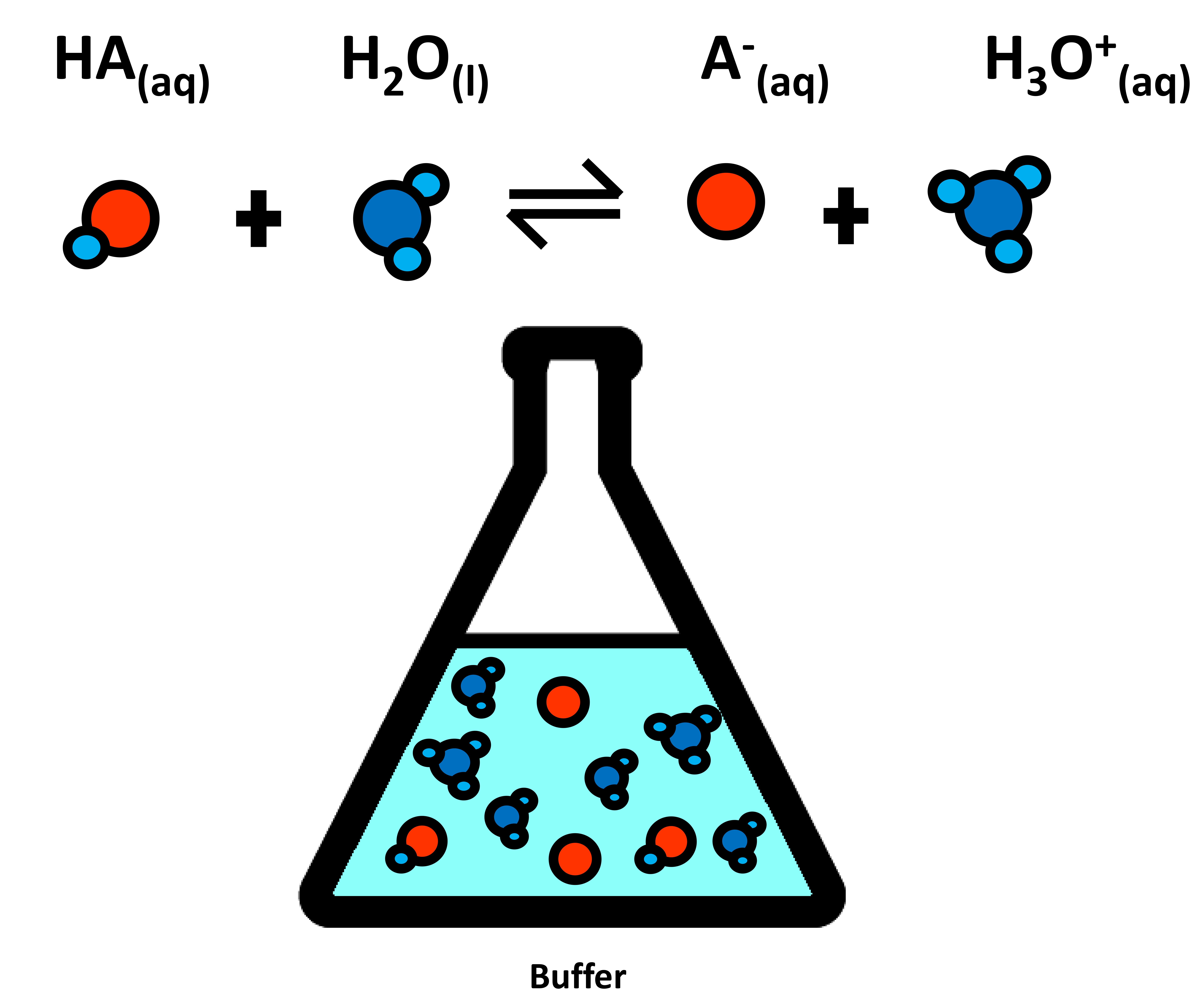 What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio
 Buffers Definition Overview Expii
Buffers Definition Overview Expii